Cleaning Validation. Official requirements
|
Here you will find answers to the following questions:
|
Cleaning procedures should be suitable to their intended purpose. This should be proved by a cleaning validation. The cleaning validation must ensure that the quality of the drug product produced using an equipment is not influenced by cross-contamination from the previous product, tenside residue or microorganisms.
The design and surface properties must be taken into consideration when facilities and equipment are constructed, so that parts which come into contact with the product are adequately accessible for the cleansing agent and are easy to dry after cleaning. (See chapter Mechanical components.)
Written cleaning procedures must be established for all equipment parts which are in contact with the product. The contents listed in figure 8.A-1 should be taken into consideration for this (see chapter Validatability of cleaning procedures).
|
Necessary contents of cleaning procedures |
|---|
|
For automated cleaning processes (CIP systems) (see chapter 1 CIP (Cleaning in Place) and chapter 8.B.3 Validating manual and automated cleaning procedures), the process parameters (e.g.: pressure, temperature, time, sequences) will have to be established in particular.
Responsible staff has to be trained on the cleaning procedures. The instructions must be locally accessible.
The critical parameters of a cleaning procedure can be determined by risk analysis. A prerequisite for the risk analysis is an established cleaning procedure with a defined cleaning objective (acceptance criteria). The risk analyses should evaluate the influence of product-, equipment- and process-specific parameters on the cleaning objective.
With regard to the product, the solubility of the ingredients, the tendency of the product towards crust formation and, where relevant, residues left by coloured ingredients should be reviewed as being critical factors to the success of the cleaning.
The equipment should be checked to see if the design (inaccessible surfaces, dead zones) or the surface materials (adsorption phenomena) could jeopardise the success of the cleaning. This check should also provide justification for the selection of the sampling points during the validation.
The FMEA method is an option for presentation of risks which should evaluate any process deviations which may occur.
- Why can a deviation occur? What is the likelihood of deviation from a process parameter? Can the cleaning procedure be designed in such a way that this error cannot occur? This aspect will be more critical with manual cleaning procedures than with automated procedures.
- Is it possible to carry out checks which are able to detect a specified deviation in time? How likely is it that a deviation will be discovered? Are corrective actions still possible at the time the deviation is discovered?
- What consequences does a deviation have on the success of the cleaning procedure or on the safety of the sequence of operation?
As a result of the risk analysis it is acceptable to combine equipment of comparable design and products with comparable chemical and physical properties in groups and to select one representative of each group as a validation object, according to a worst case approach. This selection must be justified and documented.
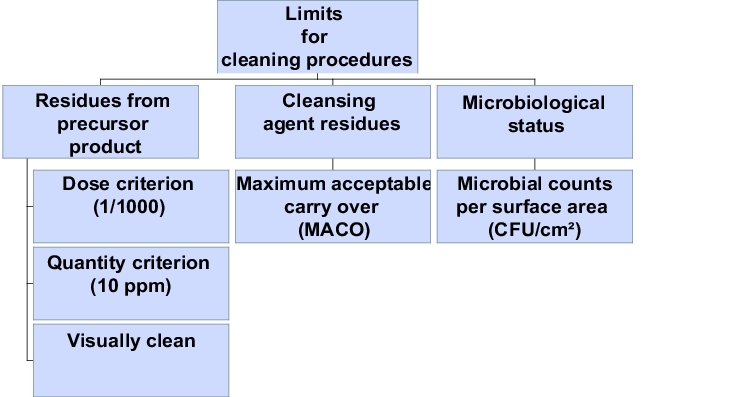
Acceptance criteria must be defined before the validation begins. For the parameters specified in figure , limits will have to be established. (See chapter Acceptance criteria and limit calculation.)
 |
Compliance with limits can be assured by direct (swab test) or indirect sampling (rinse test). The quantitative determination of residues to be carried out after sampling will require validated analytical methods. (See chapter Sampling procedures.)
The swab test (see chapter Swab test) has the advantage of absorbing residues mechanically and thus also measuring poorly soluble ingredients. However, the execution of this requires well-trained personnel (training documentation!) and the analysis involves a relatively high degree of effort (reproducibility of the process!). This method is generally suitable if the equipment parts such as agitators, scrapers, stamps etc. are accessible. A suitable material (e.g. cotton wool, filter material, bandaging material) is used to wipe a specified surface (e.g. 10 x 10 [cm2]) using a solvent suitable for the substance to be detected. After extraction, the residue can be calculated in relation to a specified surface.
The rinse test, meaning the inspection of the last rinsing water used, is limited by the solubility of the residue. The dilution of the residue can also be too high to allow a quantitative determination. This method is particularly suitable for inaccessible surfaces, e.g. valves or long piping sections. Prerequisites for the rinse test are good solubility of the product in the rinsing medium (solubility tests) and complete wettability of the equipment surfaces using the rinsing medium. If surfaces which cannot be wetted sufficiently (e.g. flanges, threads, weld seams and dead zones) cannot be excluded, it cannot be concluded from the absence of residue in the last rinsing medium that the equipment surface is free from product residue.
In addition to the methods applied for sampling, the time when sampling is carried out is also of considerable importance for its informative value. This is particularly applicable to cleaning procedures which are carried out in various stages (e.g. cleaning, rinsing, drying). Insufficient drying processes can thus lead to an increased microbial growth on the equipment. The time when the sampling is carried out is decisive when determining the number of organisms on the equipment.
The production of a placebo batch for detection of residues in the following product is not suitable as an exclusive method. It is not guaranteed that all the residues will be transferred from the equipment surface to the placebo batch. Generally, the residues are not distributed evenly within the batch. Furthermore, dilution of residues in the placebo batch may cause analytical problems.
Prior to the start of each cleaning validation a validation protocol (see chapter Documentation) must be produced, which should at least take the points considered in figure 8.A-3 into consideration. The implementation of the validation must be documented in a validation report (see chapter Documentation).
|
Cleaning validation protocol |
|---|
|
|
Cleaning validation report |
|---|
|
Changes to the cleaning procedure (e.g. composition of the cleansing agent) and the equipment to be cleaned should be assessed with regard to their effects on the validation status. A revalidation must be carried out if necessary.
|
Summary The basis of each cleaning validation is a defined cleaning procedure that is described in a cleaning instruction. Critical factors to be taken into consideration during the cleaning validation are, for example, the solubility of the precursor product, its tendency towards encrustation and the design of the equipment. Samples can be taken using swabs of a specified quality or using the last rinse water (final rinse). The implementation of the cleaning validation must be described in a validation protocol and be recorded in a validation report. |

