8.F Sampling procedures
|
Here you will find answers to the following questions:
|
According to PIC/S guideline PIC/S PI006, section 7.8, two sampling procedures are considered to acceptable:
- Direct surface sampling (swab test)
- Indirect sampling (rinse test)
Generally, a combination of the two methods is preferable, in particular if poor accessibility to parts of the equipment presents an obstacle to direct sampling alone.
The advantages and disadvantages of each method will be discussed below, as well as prerequisites necessary for their application.
8.F.1 Swab test
The swab test involves wiping a defined sampling surface (e.g. 25 cm2) with a suitable sampling material in the prescribed manner. To improve the quantitative take up of residues, the sampling material is moistened with a suitable solvent. To determine the amount of residues, the sampling material is prepared in a specified manner and the critical substance present in the eluate is analysed. Sampling is carried out preferably at the critical points of the production equipment.
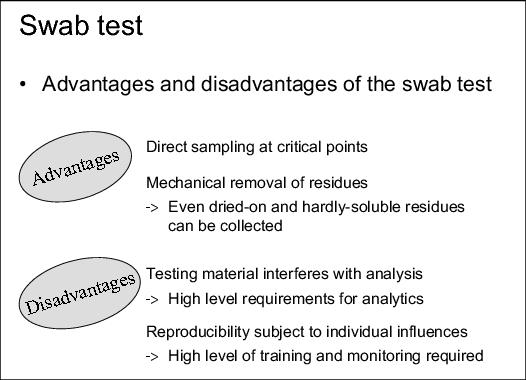
The main advantages of this sampling procedure (see figure 8.F-1) are that the sampling can be carried out directly at the critical points. Moreover, the residues are removed mechanically meaning that even dried-on and sparingly soluble substances can be gathered.
The disadvantages of this procedure (see figure 8.F-1) are that extractable components of the sampling material can interfere with the analysis, meaning that the procedure implicates strong requirements with view on the analytical method. Moreover, the reproducibility of the sampling is subject to strong individual influences. This results in a greater need for training and monitoring measures.
 |
When selecting the sampling material, several aspects must be reviewed (see figure 8.F-2).
|
Requirements for sampling material for swab tests |
|---|
|
The following can be used as sampling material, for instance:
- Cotton wool
- Filter paper
- Cellulose
- Industrially manufactured buds with a swab head made of artificial fibres
Cotton wool, filter paper and cellulose may contain impurities which could interfere with the analysis. Pre-treatment with the solvent that is to be used later to prepare the samples can remedy this.
If industrially manufactured buds are used, the swab head is usually made out of high-purity materials which do not release any further impurities. Problems can be caused however by the buds themselves, as the material they are made from may not necessarily be solvent-resistant.
Preliminary tests to check for the extraction of secondary substances are therefore strongly advised, both for sampling materials made in-house and for those manufactured industrially.
The swab test can be performed, for example, as described in figure 8.F-3.
|
Implementation of swab tests |
|---|
|
The stencil used to outline the sampling location must comply with the following requirements:
- It should be flexible (adaptable to the surface, even if it is not flat)
- It should be inert (resistant to solvents)
Foils made from PTFE, Teflon or silicone are particularly suitable. The use of adhesive strips to secure them should be avoided, as these inevitably leave residues behind when they are peeled off. In this case, it is preferable to design the edge of the stencil, such that it can be held in place by hand during sampling.
It should be kept clearly in mind when using stencils that even they can also be a source of contamination. In particular, around the edges of the defined test area, the act of wiping can produce a accumulation of residues. In this case, however, there is less risk of contamination for the equipment than for the sample, in which residues of previously carried out swab tests may occur as unwanted extrinsic peaks in the analysis. To avoid this type of problem, the stencils should either be carefully cleaned each time after they are used or only be used for a particular API.
When using silicone stencils, it should be remembered that, whilst on the one hand they have the advantage in terms of technical handling, do not require additional fixing to most surfaces and adapt well for use even on uneven surfaces, on the other hand, there is a higher risk of residue concentration, due to their porosity.
The implementation of the swab test should be documented in an SOP and practical instruction given before the cleaning validation begins.
8.F.2 Rinse test
Application of the rinse test involves rinsing the whole product contact surface of a piece of equipment or certain parts of it with a suitable solvent. To determine the amount of residues, the critical substance present in the rinsing fluid is analysed.
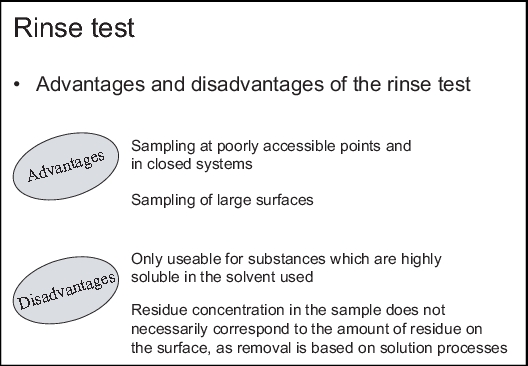
The advantages of this procedure (see figure 8.F-4) are that closed systems and parts of equipment that are hardly accessible or even inaccessible for swab sampling, can be sampled. As the sample is taken from the whole product contact surface, it is not necessary to define the critical points for sampling.
The disadvantages of this procedure (see figure 8.F-4) are its limited applicability. Firstly, the method is not suitable for all types of production equipment (see chapter 8.F.4 Selection of the appropriate procedure) and secondly, its application is acceptable only for those substances which are easily soluble in the solvent used - usually water. Officially, the objection is often raised that the concentration of residues in the sample does not necessarily correspond to the amount of residue on the surface, since the removal of the residues is only based on solution processes. It cannot be gathered from the results of the analysis, therefore, that the equipment is completely free of residues. When using organic solvents, the necessary safety measures must be taken. Moreover, large rinsing volumes result in high material and disposal costs.
 |
The prerequisites for the application of the rinse test laid out in figure 8.F-5 result from these observations.
|
Prerequisites for the application of the rinse test |
|---|
|
There are essentially two different procedures for implementation of the rinse test:
- Testing of the final rinse
- Testing of an additional rinse volume (solvent rinse)
Final Rinse
With the final rinse method, the rinsing water from the last rinsing sequence is tested. There are a series of disadvantages associated with this method:
- Its application is limited to substances that are highly water soluble.
- The sample provides evidence of the amount of residue in the final rinse, from which it is not possible to draw a direct conclusion about the amount of residue remaining in the equipment after cleaning.
- With large volumes of rinse water, the resulting residue concentration may be below the analytical limit of detection and limit of quantitation, meaning that the result is a false positive.
Reproducible results using the "final rinse" method can only be obtained if the following conditions are met:
- For the final rinse sequence always the same volume of water is used.
- The total volume of the final rinse is available for sampling (the rinsing water circulates in a closed system or is quantitatively collected).
Solvent rinse
The solvent rinse method involves sampling the clean surface with an additional volume of rinsing fluid following the final rinse sequence of the cleaning process. The are a series of advantages to this method compared to the final rinse procedure:
- The solvent can be matched to the substance to be detected.
- Sampling takes places after cleaning is completed and therefore provides better evidence in terms of the residue on the cleaned surfaces.
- The volume of solvent can be selected so that the concentration of the residue to be detected is above the limit of detection and quantitation.
Here, too, the reproducibility of the results depends on using defined volumes of solvent for sampling and on all the solvent used for the sampling being available.
The practical application of the sampling can vary greatly when the rinse test is applied, depending on production equipment and the problems posed. Figure 8.F-6 gives some examples:
|
Examples for the implementation of the rinse test |
|---|
|
Implementation for closed systems Example: Pipework, tank, blender, container
|
|
Implementation for open systems Example: Sieving machine, tablet press components
|
Due to the very variable nature of implementation compared to the swab test, it is advisable to establish only the basic requirements for the implementation of the rinse test in an SOP. Equipment-specific features during sampling (parts to be sampled, type and volume of the solvent to be used, aids, etc.) should be described in the respective validation protocol.
8.F.3 Other procedures
In addition to the sampling procedures explicitly accepted by PIC/S guideline PIC/S PI006 and by the FDA Guide to inspections of cleaning validation (FDA, 1993), the following sampling procedures are occasionally also described in connection with cleaning validation:
Steam condensate method
This method can only be used for closed autoclavable production equipment. Residue determination is carried out in the quantitatively collected steam condensate. This method can be seen as a special form of the rinse test and has the following advantages:
- Hot steam can reach all parts of the equipment.
- Hot steam possesses very good solubility properties. This method is therefore particularly suitable for lipid soluble APIs, lipophilic matrices and for cleansing agent residue.
The disadvantage of this method is that it has only limited applicability.
Placebo method
This method involves producing a placebo batch once the production equipment has been cleaned and testing for residue of the previous product.
The placebo method is described in the FDA guide to inspections of validation of cleaning (FDA, 1993) and is criticised for the following reasons:
- the residue may not be worn off the surface uniformly.
- the residue may not be distributed uniformly in the placebo batch.
- The analytical methods available may not be sufficient because of the dilution of the residue in the placebo product.
The FDA therefore considers the placebo method to be acceptable only if used in conjunction with swab tests and/or rinse tests.
Due to the low level of acceptance by the authorities and the high degree of expenditure (additional material, time and personnel requirements for the manufacture of the placebo and subsequent cleaning, more time and expense spent on equipment and methodology to prepare effective analysis) the placebo method does not play any role in practise.
8.F.4 Selection of the appropriate procedure
When selecting an appropriate sampling procedure, there are a series of aspects to consider and co-ordinate:
- the design of the production equipment (open or closed system, existence and accessibility of critical points)
- the solubility of the residue to be detected
- suitable analytical methods available
Design of the production equipment
For open systems with easily accessible critical points - following disassembly of individual parts of the equipment if necessary - it is preferable to use the swab test.
For closed systems which do not allow direct surface sampling using the swab test, it is preferable to use the rinse test.
In practice, it is not always possible to stick to the clear classification "open system/swab test" and "closed system/rinse test". It may be necessary, for instance, to carry out a rinse test on an open system (sieving machine, tablet press) to detect cleansing agent residues. In this case, it is possible, either to rinse the product contact areas with a defined volume of solvent and collect this quantitatively, or the parts of the equipment to be sampled are placed in a defined volume of the solvent and left there for a fixed period of time. The latter is recommended particularly for the testing of smaller parts of equipment.
Solubility of the residue to be detected
For detection of water-soluble substances a rinse test using water as a solvent is particularly suitable.
For detection of water-insoluble substances the swab test is mostly recommended using an organic solvent to moisten the sampling material.
Here too, in practice, it is not always possible to stick to the clear classification "water-soluble/rinse test" and "water-insoluble/swab test".
If a water-soluble residue is to be collected using the swab test, particular attention should be paid to the selection of the sampling material. While cellulose, cotton wool or filter paper can be moistened with water without problem, many artificial fibres display poor wettability. The use of water-alcohol mixtures can remedy this. Experience also shows that when carrying out swab tests with water as the solvent, a relatively high proportion of the liquid remains on the surface. In this case, it may be necessary to wipe the surface for a second time using another dry swab.
If the solubility of the residue to be verified and the design of the production equipment require a rinse test to be carried out using organic solvents, the relevant safety precautions (explosion protection, personal protective equipment ) must be taken.
Analysis
When carrying out swab tests, the samples must be prepared before the quantitative analytical determination can be carried out. The swab test offers a high degree of flexibility with regard to subsequent analysis, since during the extraction of the sampling material and the subsequent sample preparation, the requirements of/for the analysis to be conducted (e.g. solvent/mobile phase, concentration of the sample solution) can be taken into account.
When a rinse test is carried out, a sample solution is already available, which can ideally be analysed directly. Depending on the method of analysis to be employed and its sensitivity, further preparation steps may be required however (e.g. concentration or extraction).
Setting of optimum procedure parameters for sampling, sample preparation and analysis must take place as part of the development of the analytical method.
8.F.5 Microbiological testing of surfaces
The microbiological status of purified product contact surfaces is usually tested by means of the direct contact test. This test method should be presented here. (See chapter 11.E Environmental monitoring.)
Sampling material
For the direct contact test, solid culture media are used. RODAC plates (Replicate Organism Detection and Counting) consisting of a round plastic dish with a lid, the lower section of the dish holding the culture medium. This convex agar surface of 25 cm2 is pressed onto the surface to be tested. RODAC plates are very suitable for sampling flat surfaces, but are unsuitable for sampling on curved or difficult to access areas.
In addition to the round, inflexible RODAC plates, there are also flexible agar films which permit sampling of even very curved surfaces. The agar films also cover a test surface of 25 cm2.
Sampling locations
Also for microbiological testing of surfaces, sampling should preferably be carried out at the critical points of the production equipment. Those however must not necessarily be identical with the critical sampling sites for API residues. So the risk of residual moisture plays a large part in determining the sampling location. The possibility of the entry of organisms via the air or the staff during the final drying of the equipment should, however, not be forgotten . The sampling should therefore also be carried out at points which are easily accessible and are representative for the respective production equipment as a whole.
Implementation of the direct contact test
Before use, agar plates and/or films that are usually stored in a cold environment are first brought up to room temperature in a closed state. Prior to use, care should be taken to ensure that no condensed water remains in the containers To avoid contamination by the operator taking the sample, this person should disinfect their hands and disposable gloves before opening the sample container. The agar plate or film is pressed onto the area to be sampled for approximately 5-10 seconds and then immediately sealed. The sampled equipment surface is finally disinfected with 70% alcohol. This is to prevent agar residue encouraging the growth of any organism which may be present.
Other test methods
If the sampling is to be carried out at points which cannot be accessed using the agar plates or foils described, it is also possible to carry out a rinse test or swab test. In both cases, the use of sterile materials must be ensured and work must be carried out under aseptic conditions. Water or physiological saline can be used as a solvent. The sample can be smeared on a solid culture medium or be placed in a liquid culture medium.
As with the sampling procedure for determination of API and cleansing agent residues, the sampling method for determination of the microbiological quality of surfaces should also be described in an SOP and practical training given on it before the start of the cleaning validation.
|
Summary The officially acceptable sampling procedures for the detection of API and cleansing agent residues are direct surface sampling (swab test) and indirect sampling (rinse test). The swab test has the advantage that sampling can be carried out directly at the critical points and even sparingly soluble residue can be collected. The disadvantages are the high requirements for the development of the analytical method and the problem of reproducibility. The rinse test has the advantage that even closed systems, poorly accessible areas and large surfaces can be sampled.The disadvantage is that the rinse test can only be used for substances which are easily soluble in the solvent used. Furthermore, the authorities often argue that a conclusion cannot be made from the residue concentration in the sample as to the residue concentration on the surface. During the development of the test procedure, the sampling material, solvent, implementation of the test as well as sample preparation and analysis should be carefully coordinated to obtain high recovery rates. During the selection of the appropriate procedure, the design of the production equipment, the solubility of the residue to be detected and the method of analysis used should be taken into account. Microbiological testing of surfaces is usually carried out using direct contact tests. Swab and rinse tests carried out under aseptic conditions can also be used for hardly accessible sampling points. |

