Process Analytical Technology (PAT)
1 Process-analytical measurements
Process-analytical measurements are characterised by the fact that they are taken close to the process, i.e. at-line, on-line or in-line (see figure 1).
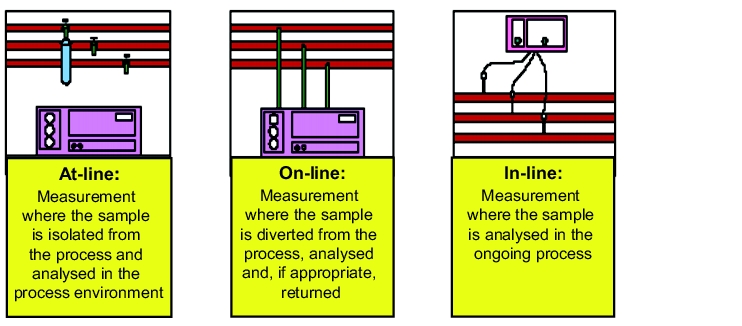 |
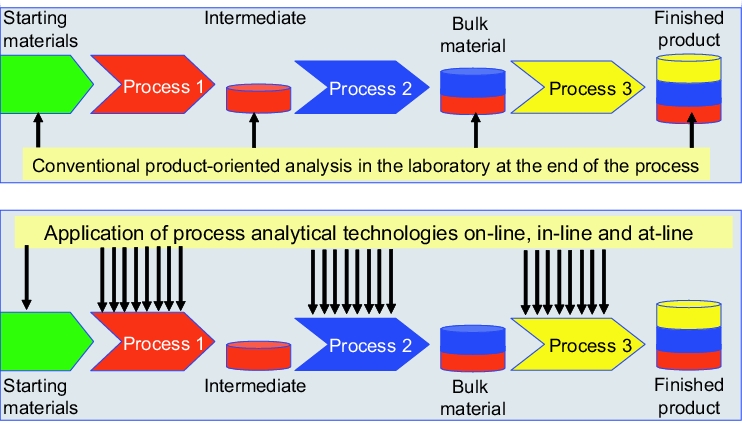
Figure 2 shows the difference in the process control between the use of PAT and conventional analysis of intermediate and finished products.
 |
PAT should not, however, be considered as an exclusively analytical instrument. It is not only the application of modern analysis technologies, such as NIR/Raman spectroscopy or acoustic measurements. PAT should also not be taken to mean that product-related investigations are simply replaced with at-line, on-line or in-line investigations (see figure 1). In fact, a clear understanding of the analytical technologies used and their strengths and limitations is an essential requirement for the application of PAT. For this, it is often necessary to employ a number of suitable, coordinated tools (see figure 3).
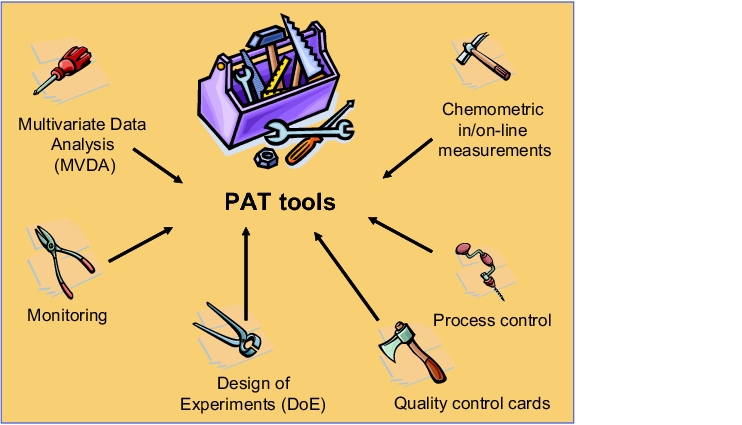 |
For example, the analytical determination of the residual moisture in a granulate is measured using suitable in-process controls (on-line or in-line) and the results are then chemometrically evaluated. The critical factors of the drying process are then determined on the basis of a statistical design of experiments (DoE) and options to improve the control/regulation of the process are thus established.
The user must therefore have a good understanding of the process that is to be checked or controlled using PAT. This requires him to know and be able to explain the causes of critical variabilities and counterregulate them with suitable process control. Finally, the PAT user must be able to predict the quality of the finished product given certain material specifications, process parameters and other manufacturing and environmental conditions. The application of PAT therefore requires a necessarily profound understanding of the process.
Nowadays, there are a large number of new analytical and chemometric options for improving pharmaceutical development, manufacture and quality control with which innovative approaches to product and process development and process analysis and control can be implemented. Systems supported by fibre optics in particular, such as FT-NIR and Raman technology, provide the possibility of direct in-process control without the samples having to be extracted or complex wet-chemical investigations having to be carried out.
2 Evaluation of the data
The considerable volumes of data that are generated when PAT is employed represent a challenge. They can essentially only be processed with powerful computer-assisted systems. The amount of data requires an appropriate evaluation using suitable chemometric tools (for an example, see figure 4).
|
Chemometric tools in NIR spectroscopy |
|---|
|
From an implementation point of view, it should be noted that the calibration models (spectrum libraries) used for comparison or standardisation purposes cannot generally be transferred from one measuring system to another, i.e. they are only valid for one factory and possibly only for one facility. The quality of the calibration model is also heavily dependent on the accuracy of the reference data, the selection of the calibration set, the individual instrument configuration (measurement mode) and the multivariate spectrum analysis (data preprocessing, regression algorithm). This limits much PAT usage to the field of large batch manufacturing in which the considerable effort associated with the creation of the calibration model is economically calculated.
3 Possible applications
In the pharmaceutical industry, there is a range of possible applications for PAT. A small selection is shown in figure 5.
|
Possible applications for PAT |
|---|
|
|
|
PAT follows the quality by design (QbD) principle, which is described in detail in the ICH Q8 document Pharmaceutical Development (see chapter E.7 ICH Q8: Pharmaceutical Development).
|
The basic principles of PAT and QbD |
|---|
|
It is an important objective to ensure that all possible types of variation that can influence a process are recognised, understood and handled by means of suitable process checking and control. This focuses on the critical product and process parameters, for which reason the ICH Q9 document Quality Risk Management should also be taken into consideration in this context (see chapter E.8 ICH Q9: Quality Risk Management ). These principles can naturally also be transferred to the manufacture of APIs.
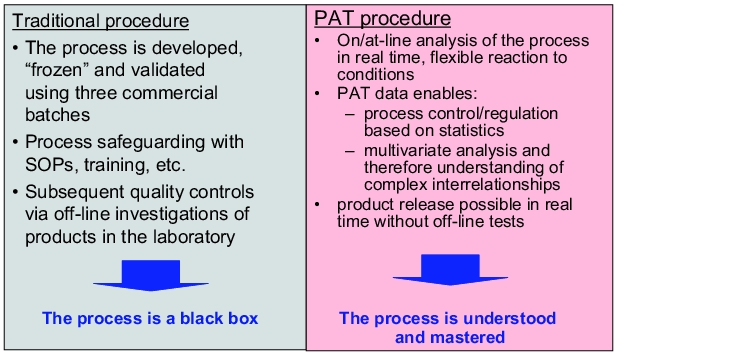 |
The consistent application of PAT can cause a paradigm shift in conventional pharmaceutical manufacturing:
Without PAT: in conventional manufacturing processes, the critical process parameters are fixed as far as possible by the marketing authorisation. As a result, the manufacturing instructions derived from the authorisation are relatively strict and allow only minimal deviations during the process cycle. Normally, pharmaceutical manufacture is carried out using batch production and laboratory tests for quality analysis are performed on selected samples (see figure 7). Changes to process parameters require the regulatory authorities to be advised of the amendment. This makes it more difficult to achieve ongoing knowledge acquisition and improvements. Because the conventional process is primarily monitored using the quality of intermediate and final products, preventative control for quality assurance is not usually (comprehensively) possible. If there is a strict process design, changes to the starting material properties inevitably result in - usually undesirable - changes to the properties of the final product, because, due to a lack of knowledge about the internal sequence of operations, material-oriented adaptations to the process parameters are not possible.
With PAT: the application of PAT allows a process to be measured and controlled in real time. Variations in starting materials and intermediates can be ideally overcome by adapting the relevant process parameters so that the quality of the final product remains the same. This means that processes can be configured in a flexible way. Granulate drying or powder mixing is not strictly ended after the specified time, but rather continues until the specified product properties or process features that are monitored and controlled with PAT have been achieved.
4 Implementations of PAT
For the implementation of PAT, relationships or dependencies between parameters must be covered by multivariate data analyses (MVDA) based on solid data (e.g. batch documentation, data from the Laboratory Information Management System (LIMS)). If historical data is not available (or not in sufficient quantities), it must be compiled on the basis of a statistical design of experiments (DoE). A procedure of this kind requires a multidisciplinary approach: representatives from different disciplines (e.g. chemometry/statistics, instrumental analysis, system technology, informatics) must work closely with each other. The sequence of operations and the product must be subjected to a careful risk analysis. The decision must be made as to which analytical system should be used and at which processing step in order to achieve meaningful monitoring. Finally, variabilities in the process should not be simulated by excessive fluctuations in the measuring system itself. For this, it will be essential to recognise all types of critical process variations and to reduce them far enough that a stable and reproducible process is achieved. The results can then be used to determine the critical PAT parameters. It is advisable to use quality control cards for the continuous monitoring.
PAT allows a number of questions to be more easily answered for the field of pharmaceutical development, such as:
- What influences the release of the medical substance?
- How do differences in the quality of APIs and excipients affect the final product quality and how does the process react to the variabilities?
- Which process parameters are important for final product quality?
5 Advantages of PAT implementation
The implementation of PAT in new manufacturing procedures or in existing manufacturing operations can bring about advantages with regard to cost and the competition.
The benefits of PAT implementation are:
- greater flexibility in process design, also from a regulatory point of view;
- a reduction in costs due to the prevention/reduction of errors, an increase in processing times and an improvement in yield;
- a reduction or elimination of end-product testing with an impact on release, meaning that the product can be released immediately at the end of the process on the basis of PAT data (real-time release);
- simplification of the initial and ongoing process validation (replacement of the three validation batches with continuous validation); and
- simplified implementation of changes to starting materials.
6 PAT in the USA and Europe
The procedure for implementing PAT brought about a need for further harmonisation, which still exists today, on the part of both industry and the authorities. This is particularly the case concerning the documentary evidence required in the authorisation process and in practice, and concerning the correlation between process measurement data and approval decisions. On the part of industry, there is sometimes the fear that the introduction of modern technologies would raise the standard of the authorisation procedure and the expectations of the regulatory authorities. In the past, this has caused industry to hold back from submitting new PAT applications, which is not a desirable outcome from the point of view of health protection and the necessity for the continual improvement of products and processes. Recognising the need to counteract this general reticence towards innovation, the FDA started a new initiative called Pharmaceutical CGMPs for the 21st Century: A Risk-Based Approach in August 2002. The FDA made PAT one of the central points of their GMP Initiative for the 21st Century and, in 2002, it founded an internal PAT working group to focus on this. In 2004, the FDA brought out its Guidance for Industry PAT - A Framework for Innovative Pharmaceutical Development, Manufacturing, and Quality Assurance, in which it sets out its ideas for the application of process-analytical technologies in the pharmaceutical industry (see chapter D.11 Guidance for Industry PAT -A Framework for Innovative Pharmaceutical Development, Manufacturing, and Quality Assurance).
At the European level, a PAT working group composed of assessors from the regulatory authorities and GMP inspectors was established by the EMEA in 2003.
The objectives of the working group include the following:
- To determine the need to adapt the rules for the use of PAT technologies and, if necessary, to develop additional guidelines for the authorisation process and for inspections.
- To define approval procedures in connection with the application of PAT
- To coordinate the sampling and investigation procedures of the official investigation centres (OMCLs) in connection with PAT
- To develop procedures for analysis during the authorisation process and inspections using models of application files for marketing authorisation for PAT applications
- To ensure a regular exchange of experiences with EDQM and FDA
At EU level, there is already an array of relevant rules for PAT which must be observed during implementation and application:
- Development of Pharmaceutics (CPMP/QWP/054/98)
- Note for Guidance on Parametric Release (CPMP/QWP/3015/99)
- Annex 17 to the EU-GMP Guideline (see chapter C.6.17 Annex 17 Final version - Parametric Release)
- Note for Guidance on Near infrared spectroscopy (CPMP/QWP/3309/01)
The EMEA PAT working group has also published a Reflection Paper, which contains information about the necessary chemical, pharmaceutical and biological data in the application file for marketing authorisation (Common Technical Document, CTD) for PAT applications. The EMEA homepage also contains a Questions and Answers section in which general interest questions about PAT applications are answered.
Industry also established PAT working groups at an early stage, e.g. the European working group of the EFPIA (European Federation of Pharmaceutical Industries and Associations) and the PAT working groups of the ISPE (International Society for Pharmaceutical Engineering) and the ASTM (American Society for Testing and Materials).
|
Summary
|

