6.C Qualification documentation
|
Here you will find answers to the following questions:
|
All qualification activities must be documented in writing as otherwise they will not be reproducible (documented evidence). The qualification documentation consists of qualification protocols, the qualification reports (see figure 6.C-1) and the qualification master plan.
|
Design qualification |
Qualification protocol |
|
Installation qualification |
Qualification protocol |
|
Operational qualification |
Qualification protocol |
|
Performance qualification |
Qualification protocol |
The structural organisation of the qualification protocols and reports is shown in figure 6.C-2.
|
Typical organisation of a qualification protocol and report |
|
|---|---|
|
Qualification plan
|
Qualification report
|
Qualification protocols and reports are brought into force/signed as follows:
- Compilation: at department level, e.g. equipment administrator and/or technician,
- Test: at department level, e.g. equipment administrator and/or technician (not identical with compiler),
- Release: at formal level, e.g. validation representative and/or head of production/quality control and quality management.
6.C.1 Qualification master plan
Annex 15 of the EU GMP Guideline only recognises the validation master plan. It may be helpful to create a qualification master plan that is subsequently integrated into the validation master plan, depending on the circumstances.
The qualification master plan may be a component of a superordinate validation master plan (chapter 7.F Validation master plan).
The qualification master plan is a useful project management tool which is used to achieve the following:
- Definition of the qualification strategy
- Description of the organisational structures and responsibilities
- Definition of the scope of the intended qualification activities
- Structuring and prioritisation of activities
- Definition of the working basis (operating procedures, internal/external guidelines, requirements for the documents (layout, doc. no., change control)),
- Definition of schedules
- Referencing and description of documents
- Definition of change control (change management/version control).
The qualification master plan identifies qualification items (= equipment) and defines qualification steps (= type and scope) for the qualification activities to be carried out for each item. In addition, the master plan describes the responsibilities during qualification.
The core element of a qualification master plan is a list of the qualification items and a schedule (figure 6.C-3).
|
Qualification |
Qualification |
Responsible |
Documentation |
|---|---|---|---|
|
Equipment item 1 |
DQ/IQ/OQ |
Qualification team |
QP01 04/03 QB01 06/03 |
|
Equipment item 2 |
DQ/IQ/OQ/PQ |
Qualification team (see protocol) |
QP02 10/003 QB02 03/04 |
|
Equipment item 3 |
DQ/IQ/OQ |
Qualification team (see protocol) |
QP03 06/005 QB03 08/05 |
Additionally, references are made to general working bases in the qualification master plan. This means that the applicable (company-internal) guidelines and operating procedures are stated in relation to the organisation/implementation of the qualification or requalification, requirements for the qualification documentation as well as deviation and change control procedures.
The qualification master plan is a comprehensive umbrella document containing references to subsequent executive regulations (operating procedures). The qualification master plan must reflect the operational circumstances and must therefore be updated if these circumstances change (e.g. new equipment).
Qualification master plans can be created as a management document for an entire site/company; it may then be useful to label this plan as a Site Qualification Master Plan. Qualification master plans can also be created especially for large projects. A higher degree of detail is then necessary, e.g. material flow, personnel flow, and the like.
6.C.2 Qualification plan
The qualification protocols (as they are referred to in the industry) are derived from the regulatory requirement for documented and predetermined specifications which at the outset define in detail the procedure and responsibilities for specific qualification tasks. The qualification protocol should describe the following contents (figure 6.C-4):
|
Content and organisation of a qualification protocol |
|---|
|
The test plans with the acceptance criteria constitute the predetermined specifications and are the core element of these qualification protocols. The acceptance criteria are defined and approved in advance and are used to assess and evaluate test results during the course of qualification activities. The test plans constitute the detailed implementation planning for the various contents of the individual qualification steps. figure 6.C-5 and figure 6.C-6 show examples of test plans.
You will find further examples in chapter 6.D.1.2 Example: Washer, chapter 6.E.1 Examples of IQ plans and figure 6.E.2, chapter 6.F.1 Examples of OQ plans and figure 6.F.2.
A comparison of both test plans reveals that a much more detailed description of what should be tested and how, as well as the quality of the testing, could be specified in the individual test plans. To avoid misunderstandings about the type and scope of the services, individual object-oriented schedules must always be the preferred option.
The qualification protocols may also be used to document the qualification activities carried out (similar to the system used for the batch production instructions - batch production record). Following implementation of the test plans, a comparison with the acceptance criteria is carried out. Deviations must be documented, evaluated and remedial measures taken. Changes made during the qualification, including the reasons for these and their evaluation, must be listed and signed. It is therefore recommended that an integral component of a qualification protocol is a running index of changes..
Test plan - "Supplier's technical documentation" |
||||
|---|---|---|---|---|
|
1 Objective The existence, completeness and factual accuracy of the documentation delivered by the supplier on the basis of the purchase requisition are checked within the scope of the test plan. |
||||
|
2 Test description The documents listed in Table 1 (target) are compared with the documents provided by the supplier (actual) to verify their existence, completeness and factual accuracy. Deviations must be documented. The documents must be referenced (drawing number and version specified) and the storage location must be indicated. The documents carry inspection marks. |
||||
|
3 Acceptance criterion All documents listed in Table 1 must be available and complete. The documents must be checked by the relevant specialist engineer to ensure that they are factually correct and must be available with date and signature as confirmation. |
||||
|
4 Test implementation and documentation Table 1: Technical documentation of supplier |
||||
|
Necessary documents |
Available |
Deviations |
||
|
Technical specifications |
||||
|
Description of the equipment |
||||
|
Operating instructions |
||||
|
Functional diagram |
||||
|
P & I diagram |
||||
|
Assembly/layout plans |
||||
|
Supply of energy and utilities |
||||
|
Technical data sheets |
||||
|
Certificates for lubricants, aids and utilities |
||||
|
Material certificates of parts coming into contact with the product |
||||
|
Necessary documents |
Available |
Deviations |
||
|
Environmental conditions |
||||
|
Servicing instructions (including intervals in operating hours) |
||||
|
Cleaning procedure |
||||
|
Calibration instruction and calibration report for works calibration |
||||
|
5 Comments |
||||
|
6 Deviations |
||||
|
7 Summary The test plan has been carried out and the accuracy of the results has been checked. |
||||
|
yes |
no |
|||
|
All acceptance criteria have been fulfilled |
||||
|
_________________________ Date/signature (implementation) |
_________________________ |
|||
IQ test plan 01: Technical documentation - supplier's P&I flow chart |
|||
|---|---|---|---|
|
1 Test item Comparison of the P & I flow chart with the completed equipment to verify existence, completeness and factual accuracy. Check to verify sufficient labelling of the equipment and check of bills of materials |
|||
|
2 Documents to be used: P&I flow chart drawing no.: xyz Bills of materials: xyz |
|||
|
3 Test description The flow chart must be checked against the existing equipment. To do this, an inspection mark (tick) must be applied to equipment components on the flow chart if they have been correctly installed and drawn. Furthermore, the bill of materials must be checked against the existing equipment and each item confirmed individually with a tick. The descriptions in the bill of materials must be verified individually. The equipment identifiers drawn in the flow chart must be attached to the facility - this must be confirmed by ticking these off in the flow chart. The apparatus bar must also be checked. Deviations must be noted on the flow chart in the bill of materials and must be checked and assessed by the Q team. The version number of the documents must be entered in the test plan. The test must be jointly carried out by two persons. The documents must be appended to the test plan and the storage location must be specified. |
|||
|
4 Acceptance criterion The documents are available, complete and checked. The facility is fully labelled. All deviations identified between the documentation and the installed equipment have been evaluated. All documents have been signed by the checkers. The documents are versioned and a new version is released by the specialist engineer responsible. |
|||
|
5 Test results |
|||
|
yes |
no |
||
|
Flow chart: xyz, version ? is available and has been fully checked. |
|||
|
Bill of materials xyz, version ? is available and has been fully checked. |
|||
|
All deviations have been evaluated. |
|||
|
No additional changes have been made to the equipment. |
|||
|
The equipment is correctly installed. |
|||
|
The facility is fully labelled. |
|||
|
The new approved version of the flow chart has been created and is available. |
|||
|
The new approved version of the bill of materials has been created and is available. |
|||
|
6 Comments |
|||
|
7 Summary The test plan has been carried out and the accuracy of the results has been checked. |
|||
|
yes |
no |
||
|
All acceptance criteria have been fulfilled |
|||
|
_________________________ Date/signature (implementation) |
_________________________ |
||
6.C.3 Qualification report
Once the qualification tasks are complete, the results obtained are summarised in the qualification reports.
Changes made to the qualification protocol or to the test plans during the qualification must be described together with a brief explanation of the reasons.
The results of the qualification must be described - e.g. all IQ test plans were implemented before the OQ, or all test plans were successfully concluded without changes and deviations.
Deviations must be described and evaluated in the report. All critical deviations that occurred within the scope of the qualification must be eliminated before the qualification report is approved. Non-critical deviations may be accepted where appropriate reasons are provided. If deviations that have not been eliminated still exist, it is important to have a tracing tool otherwise there is a danger that such unresolved points may be overlooked.
Once the qualification report has been successfully concluded, the equipment is released for use or validation and the qualification status is labelled accordingly.
The report should also formally address the following points in a summary:
- Qualification status of the facility,
- Maintenance programme,
- Recalibration programme,
- Operating instructions (SOPs),
- Document list of qualification,
- Handling of future changes and requalifications.
6.C.4 Labelling of the qualification status
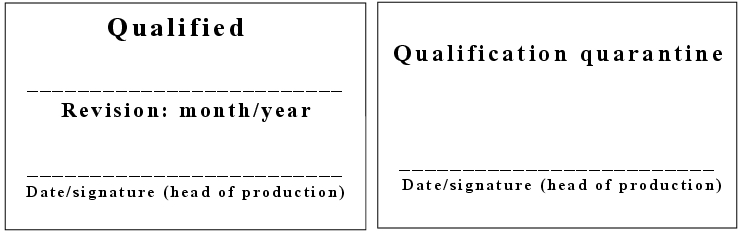
Once the qualification report has been approved, the qualification status of the equipment is labelled by inserting a qualification mark and comment in the equipment log book.
Equipment that has not yet been qualified or has lost its qualification status should be identified as such, e.g. qualification quarantine. Examples of this type of identification are shown in figure 6.C-7.
 |
6.C.5 SOP - "Qualification of facilities and equipment"
Each company should define their own specific qualification sequence in an SOP. One of many possible options is shown on the following pages.
|
Company name |
Logo |
|
Operating procedure |
SOP no. |
|
Title Qualification of facilities and equipment |
Valid from |
|
Page x of y |
|
|
Facilities |
|
|
Replaces SOP no. |
|
|
Binding for
|
|
|
For information to |
|
|
compiled by |
|
|
checked by |
|
|
approved |
|
|
Change index New compilation |
1 Introduction
Background/objectives
The qualification should verify that facilities and equipment used for manufacturing and testing are suitable for their intended purposes and that the required quality of the medicinal products manufactured can be guaranteed. Qualification is, thus, a basic factor for drug product safety.
The SOP describes the general qualification procedure.
Other relevant rules and regulations
EU GMP Guideline 3.3.4 - equipment must be suitable.
Annex 15 to EU GMP Guideline
PIC/S PI 006 - qualification, validation and cleaning validation recommendations
Commission Directive 91/356/EEC of 13 June 1991 to define basic principles and guidelines of good manufacturing practice for medicinal products for human use, Chapter II Basic principles and guidelines of good manufacturing practice, Article 8.3 3.
Definition
Qualification
Documented evidence that facilities and equipment operate faultlessly and also produce the expected results is established. Qualification is a multi-stage process consisting of the following parts:
Design Qualification (DQ)
Documented evidence that the requirements for facilities and equipment assessed in the planning phase by the future operator (user requirements) are fully taken into account in the supplier's specification (technical specification).
User requirements
Summary of all contract giver's requirements in relation to scope of supply and services.
Technical specifications
Description of implementation of all user requirments (specifications).
Installation Qualification (IQ)
Documented evidence that facilities and equipment satisfy the requirements of the design qualification in terms of identity, installation, conformity with the guidelines and documentation.
Operational Qualification (OQ)
Documented evidence that the equipment/facility is functioning correctly within the specified parameters. The operational qualification is carried out without the product.
Performance Qualification (PQ)
Documented evidence of the correct interaction of all facility and equipment components with the relevant process. This qualification phase is considered separately and is carried out by the user.
Scope and responsibilities
Person responsible for qualification
This person ensures the qualification of equipment within his area of responsibility. He appoints a qualification task coordinator and a qualification team, and also confirms that each qualification step has been concluded.
Qualification coordinator
This person coordinates the tasks of the qualification team and also compiles qualification protocols and reports.
Qualification team
The team members are staff who have specialist qualifications with regard to the technical functions; or, where computerised systems are the subject of the qualification, have specialist qualifications with regard to the relevant IT function. External companies may also be part of the team.
Quality assurance
Qualification protocols and reports are checked to verify their compliance with the rules of the quality assurance system and subsequently approved.
2 Implementation
Procurement of the equipment to be qualified is carried out by qualified suppliers.
A prospective qualification must be carried out for all new facilities and equipment. Existing equipment that is already in operation must be qualified retrospectively.
Each qualification is based on a risk analysis during which the critical parameters of the facility and the environmental conditions are observed.
The qualification scope may be confined to the operational qualification in the case of basic production and analysis equipment (e.g. pH meter or balance) and a risk analysis may be omitted.
The contents of the user requirements and technical specification may be used as the basis for the design qualification.
The technical specifications describe the purpose of the equipment and the requirements in relation to technical data and conditions
- Construction and workmanship,
- Accessories and spare parts,
- Physical and chemical parameters,
- Operation and cleaning,
- Safety at work,
- Customer service,
- Starting materials and products,
- Sampling
as well as scheduling and regulatory requirements.
Changes to the requirements are monitored by a uniform change control system during the process. A requalification is required as a result of quality-relevant changes.
The required specification describes the conversion of the equipment requirements, as defined in the user requirements, into checkable technical specifications.
The qualification is carried out and documented in the aforementioned sequence. Each qualification step is followed by a formal confirmation that the necessary qualification work has been properly completed.
Training and instruction of the operating personnel is carried out in a timely manner and is documented.
Once the qualification has been successfully completed and the equipment and facilities approved by the persons responsible for qualification, the handover is made to the user, who in turn provides confirmation.
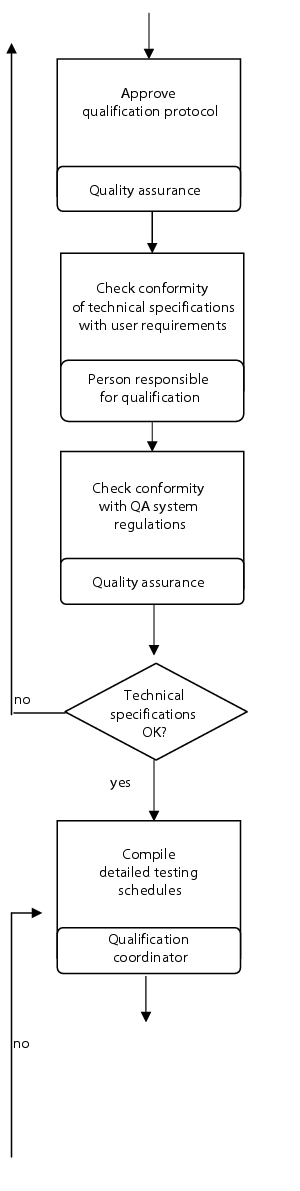
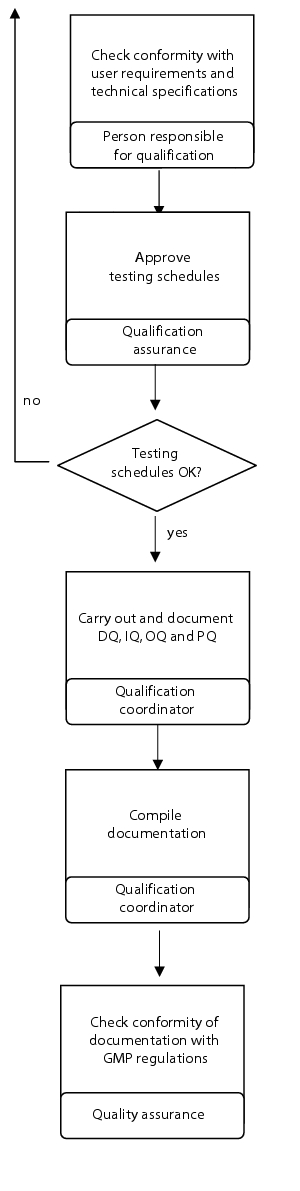
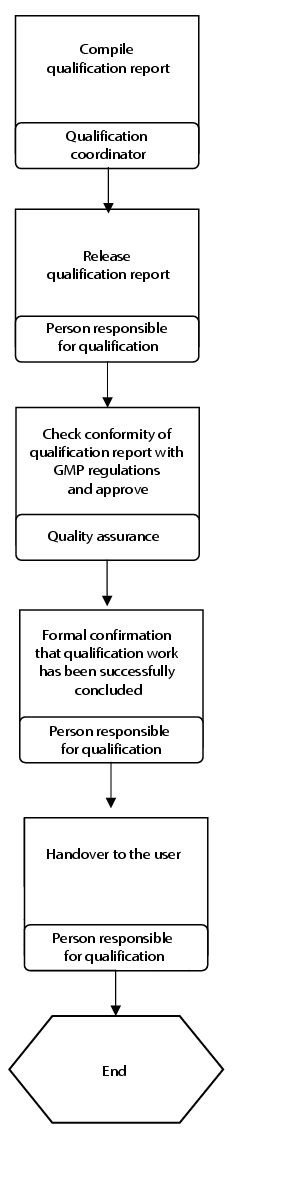
Flow chart
(See figure 6.C-8.)
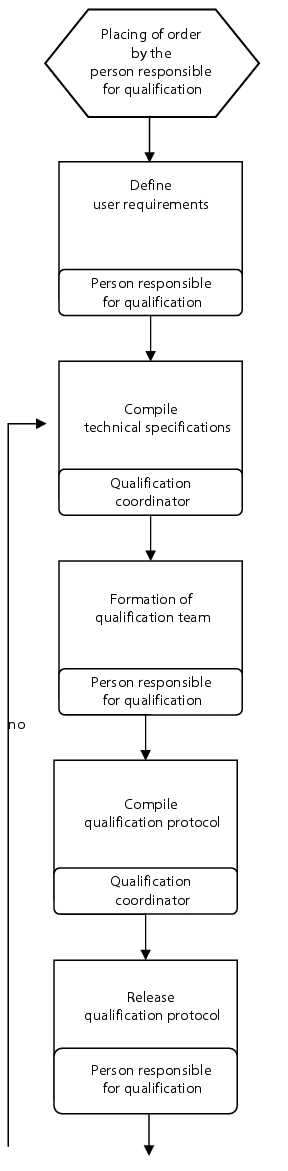 |
The qualification protocol contains:
|
 |
The testing schedules are based on the user requirements and the technical specification. Evidence of compliance with the user requirements and the technical specification is provided by the test and calibration protocols. The test and calibration documents are prepared. |
 |
Necessary documentation:
Necessary documentation for equipment that is already in operation:
|
 |
Minimum requirement for documentation of basic equipment used for production and tests:
The qualification report should at least contain the following:
|
Revision
Once the qualification status has been achieved, this is subsequently checked and evaluated every three to five years independent of the implementation of necessary requalification measures: the resulting measures and results are documented.
Documentation and storage
Once the qualification report has been approved, the qualification status of the equipment is labelled by inserting a qualification mark and comment in the log book.
The documentation should be retained for at least five years after the facility or equipment has been shut down.
|
Summary The qualification documentation consists of qualification protocols with acceptance criteria that have been defined and approved in advance, and qualification reports with the results and a final evaluation. The qualification master plan is a management instrument used to monitor all qualification activities at a company: it lists the tasks to be carried out together with the responsibilities and schedules. The general sequence is laid down in a qualification SOP. |

