Personnel hygiene
Here you will find answers to the following questions:
|
The EU GMP Guideline clearly stipulates that detailed hygiene plans must also be compiled for personnel. In addition to requirements on protective and working clothing, codes of conduct for personnel and visitors are also required. These codes of conduct are to be fixed in the form of work and organisation instructions. Different sub-areas are dealt with and discussed below.
1 Clothing
In principle, working clothing must perform different functions:
| Functions of clothing |
|---|
|
To ensure occupational health and safety, the safety clothing function is very important. Plant-specific requirements are defined here. The function of clothing as a barrier to protect the product is intended to keep back flakes of skin, the body's own bacterial flora, particles and humidity (sweat) and prevent their penetration as far as possible. The selection of specific colours for assigned areas means a signal function can be achieved, e.g. as a warning for the processing of sensitive products. These tasks are to be taken into account at the time of selection.
Clothing must be changed regularly in prescribed intervals, or even more frequently when it becomes contaminated or damaged. The higher the cleanliness grade and the greater the probability and intensity of a contamination (depending on the activity), the more often clothing must be changed (at least 1x weekly, or with grades A and B at least 1x daily). These intervals are based on the monitoring results, which should provide samples of worn clothing and fresh clothing for comparison. Figure 2 shows a comparative overview of the requirements and necessities of the different clothing, depending on the cleanliness grade. (See chapter 3.D Room classes) It is generally recommended not to implement any exaggerated clothing regulations, as there is a strong correlation between acceptance by the personnel and the insight into necessity. Packaging of the product should always be given priority over packaging of the staff.
It is recommended that an intermediate layer of clothing be worn between the sterile room clothing and personal underwear. This can help achieve a significant reduction in the release of skin particles onto the clean room clothing and then into the environment. The intermediate clothing should be antistatic and autoclavable.
| Grade |
A, B |
C |
D |
E * |
Comment |
|---|---|---|---|---|---|
Clothing |
over the entire body length (overall), sleeves and trouser legs tucked into gloves or boots |
one or two-piece, high collar, closed cuffs at wrists |
protective clothing, closed cuffs at wrists are recommended |
one or two-piece, closed cuffs at wrists are recommended |
short sleeves are not permissible |
Material |
synthetic fibres (e.g. polyamide), sterilisable or disinfectable |
cotton or blended fabric, no particle or fibre release |
cotton or blended fabric |
cotton or blended fabric |
depending on the permissible particle count or particle release |
External pockets |
not permitted |
not recommended |
not recommended |
not recommended |
to avoid mixes and cross-contaminations |
Change |
at least 1 x daily, or again with each change of area |
periodically, at least 1-2x per week |
periodically, at least 1-2x per week |
periodically, at least 1x per week |
more often in case of contamination |
Head covering |
integrated hood or neck covering (connection to over-clothing, similar material) |
fleece material change with each new inward transfer |
fleece material, changed daily |
fleece material, changed daily |
all hair must be covered |
Face mask /beard covering |
sterile, permanent wear or no beard, multiple change in each working period |
permanent wearing is recommended |
permanent wearing by men with beards is recommended |
Face mask: only when working with open products Beards: permanent coverage is recommended |
change when penetrated by humidity |
Gloves |
disinfectable, wear permanently, change with each working period |
disinfectable, permanent wearing is recommended, change with each working period |
only when working with open products |
only when working with open products |
obligatory when product is changed and when damaged |
Shoes |
covering shoes, sterilisable or disinfectable |
production shoes or covering shoes |
production shoes or covering shoes |
production shoes or covering shoes |
non-slip, but without profile due to possible product transfer |
Cosmetics |
avoid |
not recommended |
not recommended |
not recommended, not critical |
exception: pure care cosmetics |
Jewellery, watches |
avoid |
avoid |
avoid |
avoid |
also for occupational health and safety reasons |
* Grade for the production of non-sterile dosage forms, packaging |
|||||
Clothing material
In the meaning of GMP, the working clothing material must protect the products against particles and microorganisms which can be released by humans. The clothing itself must only release defined quantities of particles. As high as possible a particle retention rate must be achieved, i.e. the re-emission by the coated surface should be hampered. The specific requirements are defined by the target cleanliness grade in line with the available rooms and facilities (see figure 3 and figure 4). The design of the facilities should therefore be included in the risk assessment. Completely closed systems allow for a different clothing quality to be used than systems that expose the product to the environment temporarily.
| Criteria for the choice of material |
|---|
|
In contrast to the requirements for the production of sterile preparations, the cleanliness classification does not assert any requirements regarding the particle count in the production of drugs for oral application. In terms of the microbiological purity, on the other hand, limits for preparations are defined in the European Pharmacopoeia. It is therefore advisable, even for the production of non-sterile drugs, to establish requirements for the particle release by the material and cleanability.
For sterile forms, pure synthetic fibres such as polyester or polyamide (area A, B) or mixed fabrics with cotton are usually used. Here, trapped heat and perspiration make the material uncomfortable to wear. A higher proportion of cotton in fabrics makes the material more comfortable, but releases more particles. It should be possible to disinfect the different fabric types depending on their application, and clean room clothing should be sterilisable e.g. autoclavable. It must be ensured that objects such as closures are not affected during application of disinfectant in the preparation process.
Design of the clothing
The design chosen for the clothing is important for its functionality and its acceptance by staff.
| Requirements for design selection |
|---|
|
- Cut of the clothing
The clothing must be appropriate for the activity (see figure 2). This also presents requirements in terms of practical manageability. For working steps in sterile preparation, there are explicit clothing requirements. It must cover the full length of the body (one or two-piece) and cover the neck, e.g. as an overall with a hood or a neck covering (see figure 9). For lower cleanliness grades, coverage of the complete body area is not necessary (see figure 10 and figure 11). Only exposed areas must be covered. The clothing must be closed at the wrists, e.g. through elasticated cuffs. The length of sleeves and trouser legs should be designed so that no uncovered areas remain when gloves and shoes are worn, e.g. if the sleeves are too short. Closure at the wrists enables gloves or arm cuffs to be rolled over, so that the lower arm is not uncovered. In addition, this helps prevent the transfer of particels or product through unwittingly carrying around in open sleeves (see figure 5 and figure 6). Personnel often roll their sleeves up during cleaning work with extensive washing activities in non-aseptic areas. In order to protect product contact surfaces, the use of water-repellent materials should be provided for here, e.g. through long-cuff gloves. Through individual assignment, the correct fit of the clothing can be guaranteed for the employee in question. This means that the clothing must be uniquely identifiable, e.g. through sewn-in barcodes, numbers or the name of the employee. - Putting on
It must be possible to put the clothing on with a minimum amount of contamination. In clean rooms working cloths should be changed before re-entering. Surface microbial count determinations show that the exterior and interior both become contaminated depending on the wearing time, intensity of movement and perspiration. In addition, it is barely possible to remove and hang up the clothing without contaminating the exterior.
When putting the clothing on, it is greatly beneficial to have an adequate mirror to ensure the correct position of the clothing. This also applies for areas in which face masks or head coverings are worn. - Gloves
In the field of sterile production, un-powdered gloves (possibly gradiation sterilised) are used to prevent possible particle loading. The cuffs of the overalls are tucked into the gloves to avoid leaving uncovered areas. For safety, additional sterile sleeve cuffs can be used which cover the sleeve/glove connection area.
Gloves are to be changed immediately if they become damaged, and after they have been used to touch non-sterile objects, as when lifting objects that have been dropped. Unnecessary contamination (e.g. through scratching, making telephone calls) is to be avoided. Entry into a critical area always requires disinfection of the gloves. For use in grade A and B, gloves should be treated with disinfectants which are sterile at the time of application. It must be ensured that the disinfectant is compatible with the material of the glove. Otherwise, latent penetration with maceration effects may occur. It is recommended to aim to co-operate with the manufacturers of disinfectants in order to take advantage of their experience and avoid errors. The use of 70% isopropanol is usually adequate for disinfection. The pharmaceutical manufacturer verifies and evaluates the system so that there is no dependency on subjective supplier recommendations.
Figure 5
Clean room clothing with sleeve cuffs
Figure 6
Class E clothing with inadequate sleeves or gloves - External pockets
External pockets are not permitted in clean rooms. On the one hand they hold the hazard of accidental cross-contaminations, and on the other hand the unintentional bringing of non-sterile or non-disinfected objects, e.g. pen, tissues, into the clean room. In principle, this requirement can also be applied to manufacturing in lower cleanliness grades down to non-sterile drugs. In this case, the problem is primarily the involuntary carrying of parts or products with subsequent mixing. Therefore, there should be no external pockets on clothing in these areas either. - Hair coverings
The head coverings must be complete, i.e. the hair must be completely covered. For work in non-sterile areas, fleece material is usually used, while in cleanliness grade A-B the same material is sometimes used as for the suits. The head covering is integrated in the suit or must be tucked into the collar (see figure 7).People with very long hair sometimes have to wear two head coverings (fleece fabric) at the same time to ensure complete coverage. One of the management's tasks is to ensure that the head coverings used are suitable for all employees. Consistent execution, i.e. the same rules for managers and visitors, is of absolute importance.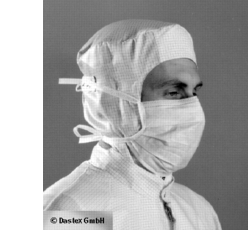
Figure 7
Example of coverings in clean rooms
The problem of beards and moustaches in sterile areas is dealt with explicitly. The risk of releasing particles is accounted for with the requirement to cover or remove the beard. For non-sterile areas, the correct manner of wearing the beard shield is also important. It may be that glasses are required in clean rooms to protect the products against the employees' uncovered eyes (e.g. eyelashes). The individual necessity of this measure must be checked during risk analysis. Apparative devices, such as air flow control (e.g. laminar flow) may make this unnecessary. This does not affect the safety aspects of wearing glasses. - Mouth mask/face mask
The mouth and nose are covered by masks. These masks are to be changed periodically (at the latest when penetrated by moisture), to prevent a critical penetration of microbes.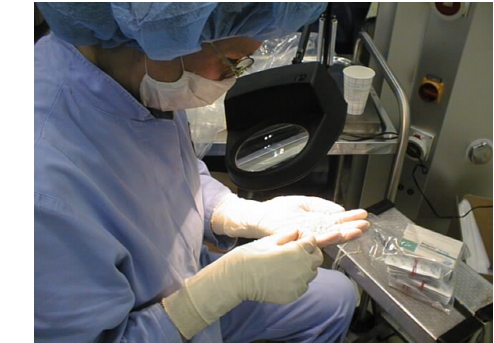
Figure 8
Coverings when working on the open product (grade E)
As the mask is only effective when dry, employees must only remain in the sterile area for as long as is absolutely necessary. The masks used consist of various materials (integration in the hood fabric, multilayer fleece material, paper, etc.). It is also important to wear the face mask in non-aseptic areas. In these areas, it is not generally necessary to cover the mouth, nose and hands. However, these measures are essential when working on open products and on product contact surfaces (see figure 8). In this case, it is important to define these activities clearly in the practical workflow and demonstrate them to the staff in a comprehensible manner. The analysis of production processes or individual working steps enables precise specification of the measures for each activity. For practical reasons, open products are to be avoided as far as possible by using closed containment, as this can significantly reduce the effort required, i.e. putting on, wearing and removing masks or gloves. - Shoes
In principle, the same requirements apply for shoes as for the clothing material. Overshoes should, as far as possible, be tearproof (especially single use shoes). Here, the GMP requirements must be brought in line with the safety requirements (e.g. anti-slip). It should be possible to clean the shoes. This can be done, for example, using a sole cleaning machine.
For sterile areas, the leg openings of the clothing must be as tight as possible and must be tucked into the overshoes. For grade A and B, it must be possible to sterilise or disinfect the boots.
Figure 9
Example of clean room clothing
Preparation/Cleaning
Working clothing is only used as single-use clothing in exceptional cases, as the costs for this are usually very high. Therefore, processing steps are carried out to prepare the clothing for reuse. The processing can be carried out within the plant or can be outsourced as a service. In the field of sterile manufacturing, a number of requirements must be taken into account. The ageing process of the clothing caused by cleaning processes must be taken into consideration, as this could result in a higher release of particles. There is no need for separate processing of clean room clothing for non-sterile manufacturing. The logistical organisation, i.e. responsibilities and processes, such as collecting, checking, dispatching, dispensing, etc. must be regulated. In addition to checking the number of particles in the fabric, microbiological testing should also be carried out after preparation. For quality assurance purposes, the service provider should be inspected and evaluated as part of the supplier qualification. The reduction in microbes through the preparation procedure is reviewed during monitoring.
There are different processing requirements:
- The preparation of the clothing must remove the particles released by people (dandruff, etc.).
- Production dust must be removed in order to avoid cross-contamination. Different items of clothing contaminated with different products should not be cleaned together. This is to be considered in light of the potential risk.
- Preparation must be carried out as gently as possible so that no changes are caused in the fabric. This also helps achieve longer usability of the clothing.
- A visual inspection is carried out for wear and tear: thin spots, damage, condition of the seams.
- The preparation steps must be executed in a defined manner, i.e. reproducibly. Temperature, time and cleansing agent are defined by type and quantity. The exact processes (e.g. flows) are prescribed in order to avoid secondary contamination from external areas, for example. Drying, packaging and possibly sterilisation and storage are defined.
Special requirements for clean room clothing:
- Keeping a kind of log or identification and traceability by means of barcodes, which are applied to the inside, is recommended.
- Via single part tracking, it is possible to assign batches to cleaning and sterilising processes with the associated documentation.
- An usability date should be documented for sterilised clothing in order to avoid excessive storage periods. This is especially important for reserve clothing with an unspecified storage time, e.g. in visitor locks.
- In the case of external preparation, the clean, decontaminated and sterilised clean room clothing should be heat-sealed air tight and returned in closed boxes. Quality control before heat sealing can be carried out with laser particle counters.
- Disinfection of the laundry can be carried out in washing machines in combination with drying under a laminar flow (for clean room clothing that cannot be sterilised in autoclaves).
Gowning procedure
The hygiene procedure includes a clear, area-specific definition of gowning. This must be logical and consistent. It should also be coordinated with the structural circumstances so that different areas which require a change of clothing can be easily identified as such. The area assignment can be visualised by using pictograms or colour codes. Here, different cleanliness areas across the plant can be identified via specific pictures or colours on walls or doors. Imprecision in terms of unclearly defined areas is to be avoided for acceptance reasons. Passage between areas is via personnel locks. The exact procedure for the transfer between areas and the associated clothing change is described in process descriptions. These processes are to be discussed in-depth in training courses, in order to achieve the necessary understanding and motivation of the staff. Compliance should be permanently monitored.
It goes without saying that a change of activities on different products is critical in terms of clothing. As re-entry into clean rooms requires a change of clothing in any case, this is of particular importance for non-sterile areas. Activities that involve such a change should be examined separately during the analysis and a precise procedure should be established.
In addition to the production staff, visitors and service employees must also be provided with the appropriate clothing. For sterilised qualities, the sterility expiration date of the stored clothing must be noted.
2 Personnel hygiene
Employees are expected to maintain a high level of personal hygiene. This is understandable, as existing hygiene plans are based on defined average values of microbial counts. Therefore, care of finger nails, hair and skin must be defined. In addition to a disproportionate increase in the microbial count if personal hygiene is neglected, there is also the hazard that finger nails could damage the gloves and clothing.
Regular personnel training and testing should make personnel aware of the significance of personal hygiene. It can be advantageous for the company to provide utensils such as soap and towels, in addition to sufficient washing and cleaning rooms.
3 Code of conduct
Codes of conduct are prescribed to ensure a defined cleanliness grade in terms of the particle loading and the microbial status.
| Critical behavioural aspects |
|---|
|
- Food, cigarettes, jewellery and other personal objects (e.g. newspapers) must not be brought to the place of work. Eating, chewing, drinking and smoking is strictly forbidden in the production areas. Suitable recreational rooms must be provided for this purpose. The transition between areas is to be consistently via a lock, in which the production clothing is changed or (depending on the production cleanliness grade) at least sufficiently covered.
- Employees working in clean rooms should - at least during their total working time - not smoke. The time dependency between particle release with exhaled air and the rest time since smoking has been proven. Only approx. 15 minutes after finishing smoking is the average particle release value of non-smokers achieved. This is demonstrated very effectively in training courses.
- Before entering the production area, openly worn jewellery and watches must be removed. This is also required for safety at work.
- Cosmetics must be used sparingly or preferably not at all by people who work in clean rooms. The hazard of contamination must not be underestimated. A stipulation of the absence of any cosmetics avoids the gradual grading of permissible and non-permissible quantities. Particle release is to be graded as critical.
- In clean rooms, speaking should be kept to an absolute minimum in order to prevent accelerated humidification of the mask with a penetration of microbes. Coughing and sneezing must not be directed towards the product. This can result in direct contamination. Raising awareness during training can clarify the hazard potential.
- Only the absolutely necessary number of people should work in clean rooms at any one time. Staff who are not involved in the manufacturing process should leave the clean room. The most effective way to minimise the number of employees in the sterile area is to execute only the absolutely necessary production steps in clean rooms. Activities such as coding ampoules or visual checks on products can be carried out outside the clean rooms in lower cleanliness grades.
- The movements of people in clean rooms should be calm and uniform as this will have the least negative effect on flow ratios and causes the lowest release of particles. In any case, movement should be as deliberate as possible. Simply folding your arms can lead to contamination of your gloves. The hands are placed near the armpits which, even on the outside of the fabric, are the areas with the highest microbial count. (Hecker, p. 70)
4 Hand disinfection
Before starting any activity in the production area, the hands or gloves must be disinfected regardless of the type of production. As microbes are continuously reproducing, periodic disinfection must also be carried out. The frequency of this measure depends on the cleanliness grade, the activity and the technical circumstances (closed or open systems). The frequency and exact method of execution are to be fixed in writing in an operating procedure. The disinfectant manufacturer's specifications are to be taken into account (for example, see figure 13). In any case (even for non-sterile production), the hands must be disinfected after going to the toilet or eating, drinking or smoking.
| Carrying out hand disinfection |
|---|
|
Hand cleaning and disinfection solutions are available individually and as combined products. The design of the facilities in the washing area should enable operation without using the hands. This means considering the process with all individual elements: Control of the water supply (e.g. activation via motion sensor, foot operated switch), dosage of the cleansing agent (elbow lever), drying (paper towels or hot air dryer), waste disposal (pedal bins), etc. The use of hand towels and bars of soap should be avoided. Most disinfectants are applied as rub in disinfections. This means that the disinfectant is rubbed into dry hands for a certain period of time. The disinfectants used should be approved by the respective national test centre. There must be an adequate number of dispensers for disinfectant solutions. This will avoid long waiting times in the current work process and will increase acceptance levels. As part of monitoring, the microbial status of the cleansing agent and disinfectant dispensers must be regularly reviewed.
Pictograms or clear instructions are helpful. This is important for visitors or external workers who are not familiar with these procedures.
5 Health requirements
Only persons who do not have any infectious diseases at the time of production may be employed to manufacture drugs. This is ensured through health monitoring. (See chapter 2.B.2 Health requirements.)
6 Training
For the field of hygiene in particular, training courses represent an essential basis for the necessary measures. Without correct explanation, sufficient compliance with the guidelines by the employees cannot be counted on. This results in inconsistent, partly incorrect handling of the requirements. In the field of sterile production in particular, the smallest microbial contaminations are extremely critical by nature. In this respect, dialogue between the management and production employees must be sought. In cases of doubt, unclear formulations in the requirements and unclear labelling of rooms can lead to different interpretations by the employees, without carelessness. In this dialogue, the principles of microbiology can be communicated and the requirements can be explained. The involvement of the staff in the selection of working clothing, e.g. as part of a test phase, can give an indication of the practical suitability of the clothing. In addition, a high level of acceptance by the staff can be achieved. (See chapter 2.C Training.)
Summary The working clothing requirements are directly linked to the cleanliness grade in which production is being carried out. Cleanability, particle release, suitability and wearing comfort must be taken into account when selecting the clothing. The clothing must be cleaned (prepared) in accordance with an established procedure in order not to affect the quality of the material. For each cleanliness area, there should be a gowning procedure which is taught intensively. Personnel hygiene includes the health check and training on special codes of conduct. |



