Active Pharmaceutical Ingredients
1 Objective
Historical Background
When the initiative was taken by PIC/S at the Canberra meeting in September 1996 to draft a globally harmonised Good Manufacturing Practices (GMP) guide for the Production of Active Pharmaceutical Ingredients (APIs), the recommendation was made that this should essentially be a "what to do", rather than a "how to do" document.
After that initiative the International Conference on Harmonisation (ICH), which consists of the three major pharmaceutical regions of the world - USA, Japan and Europe - took the topic on board. The ICH established an Expert Working Group (EWG) which membership was due to the importance of the topic extended beyond the three regions to WHO, PIC/S members, India, China and OTC, Generic industry and CEFIC/APIC representatives. The EWG has compiled the "GMPs for APIs" Guide within 2,5 years time. The document was finalised by November 2000 and is now implemented within the three regions.
Purpose of the ICH Q7a Document
Industry should avoid needless paperwork and administrative burden. As indicated in the Q7a document the focus should be - for the benefit of the patient - on identifying the critical controls and procedures that assure the quality of the API. Therefore, sound scientific judgement should prevail when setting up a quality system incorporating GMP. The word "should" is extensively used in the final version of the ICH Q7a Guide. It indicates requirements and recommendations that are expected to apply unless shown to be inapplicable or replaced by an alternative that can be shown to provide at least an equivalent level of quality assurance. Hence, "should" does not mean that because it is only a "should", and not a "must", then this requirement does not have to be met.
Regulatory Requirements
Companies should be aware that the regulatory filing requirements might differ from the application of GMP as defined by Q7a. There may be cases where more information may be required by regulatory authorities, but inspections for compliance with the Q7a Guide should only cover the GMP relevant steps.
Scope
API Starting Materials
| Definition of API starting material (ICH Q7A 1.3, see chapter E.6 ICH Q7A: Good Manufacturing Practice for Active Pharmaceutical Ingredients) |
|---|
An "API Starting Material" is a raw material, intermediate, or an API that is used in the production of an API and that is incorporated as a significant structural fragment into the structure of the API. An API Starting Material can be an article of commerce, a material purchased from one or more suppliers under contract or commercial agreement, or produced in-house. API Starting Materials normally have defined chemical properties and structure. |
Companies are responsible for proposing the API Starting Material(s). This is one of the most significant proposals in the ICH Q7a document. The technical and quality departments should work closely with regulatory departments to ensure no disagreement occurs on the proposed starting materials. Ideally the registration of New API's will start from the same Starting Materials defined from a GMP perspective. However, based on current regulatory requirements it is likely that the regulatory authorities will require further information on API Starting Materials where only one or two synthetic steps exist between the API starting Material and the API or where the API Starting Material is an API itself.
The companies should review the synthetic process of each API and based on technical and quality assessments define what are the significant structural fragments beyond which the GMP standards defined in ICH Q7a should apply. In general, the source of the API Starting Materials is not the major factor.
EP Certification submissions require that a statement is made that a product is manufactured according to GMP. This declaration does not apply for an "API Starting Material" described in a Pharmacopoeia. Clearly, this may be conflicting with the Q7a definition on API Starting Materials.
The regulatory authorities may also require further details for late stage API Starting Materials, though recent examples are known that final intermediates as API Stating Materials (e.g. widely commercially available substance or 6-APA for the manufacture of semi-synthetic penicillin's) have been accepted.
Guidance on how to define API Starting Materials
- Follow the guidance given in ICH Q7A and involve technical, quality and regulatory departments in agreeing the definition of the API Starting Materials. Where possible use the same definition of API starting material in regulatory filings and in defining the steps for which the GMP requirements of ICH Q7a apply.
- Further guidance on how to define the API Starting Materials and regulatory strategy is given in
- "The Active Pharmaceutical Ingredients Starting Material (APISM) and other materials in API manufacture: Scientifically-based principles for the Common Technical Dossier" by Helga Möller and Chris Oldenhof, Drug Information Journal, Volume 33, Number 3, 1999, pages 755 - 761 and
- Eudralex Vol. 2b, page 162 ("Validation of the process should be carried out ... for steps of the manufacturing process which are critical for the product")
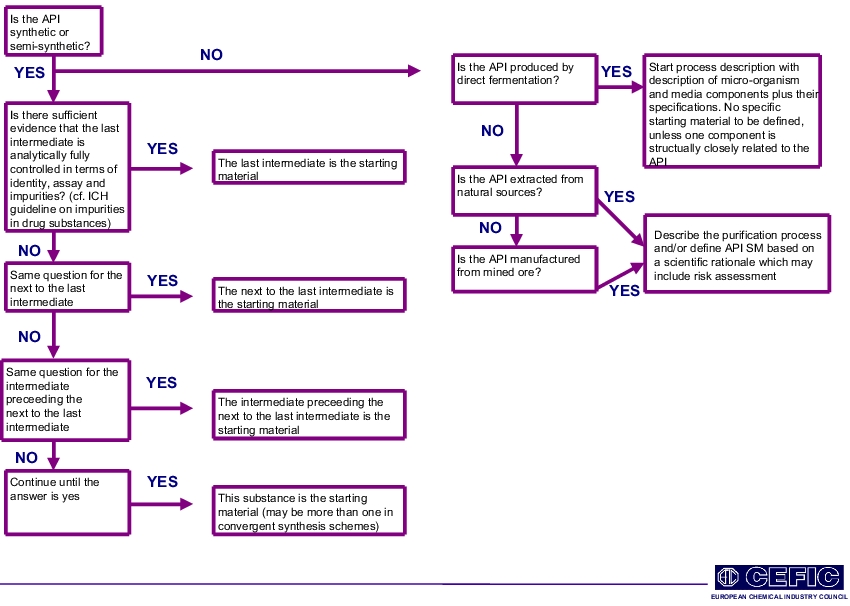
- The API Starting Material Decision Tree, developed by CEFIC/APIC and FIP, is the central feature of this guidance (see figure 3).
- Where the proposed API Starting Material is close to the API itself ensure that details on the synthetic process and analytical controls used to manufacture the API Starting Material are available in case these would still be (justifiably) requested by the regulators. Where the API Starting Material is a commercial molecule the requirement to provide these details (if needed for confidentiality reasons: directly to the authorities only) may be included in the commercial contract.
- Similarly, Change Control requirements should be defined in the commercial contract for supply of API Starting Materials. Any significant changes to the synthetic route, analytical controls or specifications by the manufacturer of the API Starting Materials in general needs notification to and acceptance by the API manufacturer.
- While API Starting Materials do not require to be manufactured to the GMP requirements defined in ICH Q7a, manufacturers of intermediates and/or API's should have a system for evaluating the suppliers of critical materials (Reference Q7a Section 7.11 - chapter E.6 ICH Q7A: Good Manufacturing Practice for Active Pharmaceutical Ingredients). Appropriate qualification of API Starting Material suppliers is required.
Companies should consider redefining the API Starting Material for well-established products. This offers the opportunity to reduce the overall GMP requirements for early manufacturing steps and to shift the focus to be on the control of the critical synthetic steps starting from the redefined API Starting Materials. Any proposed re-definitions to API Starting Materials should of course be agreed with the regulatory authorities. The FDA have already indicated their willingness to reduce the filing requirements for certain well established "Qualified Products", including those relating to the final API synthesis steps.
| Type of |
Application of this Guide to steps (shown in grey) used in this type of manufacturing |
||||
|---|---|---|---|---|---|
Chemical Manufacturing |
Production of the API Starting Material |
Introduction of the API Starting Material into process |
Production of Intermediate(s) |
Isolation and purification |
Physical processing, and packaging |
API derived from animal sources |
Collection of organ, fluid, or tissue |
Cutting, mixing, and/or initial processing |
Introduction of the API Starting Material into process |
Isolation and purification |
Physical processing, and packaging |
API extracted from plant sources |
Collection of plant |
Cutting and initial extraction(s) |
Introduction of the API Starting Material into process |
Isolation and purification |
Physical processing, and packaging |
Herbal extracts used as API |
Collection of plants |
Cutting and initial extraction |
- |
Further extraction |
Physical processing, and packaging |
API consisting of comminuted or powdered herbs |
Collection of plants and/or cultivation and harvesting |
Cutting/comminuting |
- |
- |
Physical processing, and packaging |
Biotechnology: Fermentation/cell culture |
Establishment of master cell bank and working cell bank |
Maintenance of working cell bank |
Cell culture and/or fermentation |
Isolation and purification |
Physical processing, and packaging |
„Classical" Fermentation to produce an API |
Establishment of cell bank |
Maintenance of the cell bank |
Introduction of the cells into fermentation |
Isolation and purification |
Physical processing, and packaging |
|
|||||
|